Good Manufacturing Practices (GMP) are foundational principles in the food industry, designed to ensure the quality and safety of food products. By setting standards for every stage of food production—from receiving raw materials to the final shipment—GMPs aim to prevent contamination, cross-contamination, and the presence of foreign substances. This article explores the importance of GMP, its guidelines, and the benefits of GMP compliance.
What is GMP?
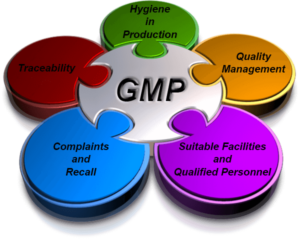
Good Manufacturing Practices (GMP) are defined guidelines that establish quality control standards for manufacturing processes, ensuring that products are consistently produced and controlled to meet high-quality criteria. Unlike final product testing, which cannot always guarantee safety, GMP standards proactively address all aspects of production. In the food industry, GMP extends to raw materials, facilities, equipment, and the personal hygiene of staff.
Importance of GMP in Food Production
In the food industry, GMP compliance is vital for maintaining safety, meeting regulatory requirements, and protecting consumer health. The significance of GMP can be summarized through these key aspects:
Ensuring Food Safety: GMP protocols ensure that food safety standards are met at each production stage, systematically controlling processes to minimize contamination.
Compliance with Regulations: GMP compliance is often legally required, ensuring companies avoid penalties and meet standards set by authorities like the FDA.
Consumer Confidence: Products manufactured under GMP standards tend to inspire greater consumer trust, fostering brand loyalty.
Market Access: Many markets require GMP certification, making it essential for companies to access and compete in broader markets.
Cost Efficiency: By reducing recalls and operational inefficiencies, GMP implementation often results in significant cost savings.
These benefits underscore that GMP is not only about regulation but about creating a culture of food safety and quality.
Key Guidelines for GMP in the Food Industry
For manufacturers, GMP guidelines cover several areas critical to maintaining food safety and quality:
Documentation and Record Keeping: Accurate documentation and record-keeping are essential to GMP, providing a verifiable history of all production activities. Key documentation includes:
- Batch Records: To track production specifics and ensure traceability.
- Cleaning and Maintenance Logs: Verifying that equipment meets sanitation standards.
- Personnel Training Records: Ensuring staff competency in GMP standards.
- Quality Control Results: Recording testing outcomes for quality assurance.
This meticulous record-keeping supports transparency and demonstrates adherence to GMP requirements.
Personnel Hygiene and Training: Personnel hygiene is crucial in preventing contamination. Staff training should cover essential topics, such as handwashing, illness reporting, and allergen management. Training should be continuous and documented to keep all staff up-to-date on GMP practices. Effective training programs reinforce food safety and ensure everyone understands their role in maintaining GMP standards.
Facility and Equipment Maintenance: GMP requires facilities to be designed for cleanliness and safety. Regular equipment maintenance, pest control, and calibrated cleaning schedules help minimize contamination risks. Adherence to these standards ensures that both facilities and equipment support safe and efficient production.
Implementing GMP in Food Manufacturing
GMP implementation involves establishing detailed procedures for all stages of production:
- Standard Operating Procedures (SOPs): Guidelines for each critical process to ensure uniformity and safety.
- Continuous Staff Training: Ensuring staff understand and execute their roles in food safety.
- Environmental Monitoring: Regular checks to ensure the manufacturing environment remains contamination-free.
- Process Control: Monitoring production variables to guarantee consistent product quality.
GMP also includes strict quality control and assurance measures. These processes help detect and correct quality issues, ensuring products consistently meet safety standards.
Auditing and Compliance
Regular audits, both internal and external, help manufacturers assess and maintain GMP standards. Audits verify compliance with national and international regulations, while also identifying areas for improvement. Staying current with regulatory changes is also essential, as GMP standards evolve to address new food safety challenges.
Benefits of GMP in the Food Industry
The adoption of GMP in food production delivers numerous advantages that contribute to both business success and consumer safety:
Enhanced Food Safety: GMP’s rigorous control measures reduce contamination risks, helping prevent foodborne illnesses and protect public health.
Regulatory Compliance: Meeting GMP standards is often mandatory. GMP compliance demonstrates that companies meet regulatory requirements, avoiding legal issues and costly recalls.
Increased Consumer Confidence: Products manufactured in GMP-certified facilities inspire trust among consumers, who recognize the company’s commitment to safety and quality. Displaying GMP certification on products reinforces this trust and enhances brand reputation.
Conclusion
Good Manufacturing Practices are foundational in the food industry, fostering a culture of safety, efficiency, and consumer trust. By adhering to GMP standards, food manufacturers not only meet regulatory requirements but also demonstrate their dedication to quality. For companies seeking to thrive in today’s market, GMP implementation is a strategic investment in both consumer trust and long-term success.
 Food Manifest
Food Manifest